
How to get your digital therapeutics on the USA market?
12 min read
Contents
Have you manufactured a digital therapeutics (DTx) and want to market it in the USA? It’s possible. There are many ways of achieving this goal, and in the following article, we will tell you all you need to know about introducing your DTx in the USA.
TL;DR
In this article, we focus on rules the FDA (U.S. Food and Drug Agency) states that allow manufacturers to have their medical devices marketed in the USA.
We define the differences between FDA listed, FDA cleared, FDA approved, and FDA granted. You will also learn about ways of getting your DTx on the American market, such as Premarket Notification 510(k), Premarket Approval, and De Novo Classification Request.
Moreover, we focus on the data safety standards demanded by the FDA, describe the DTx reimbursement scheme, and show you the challenges of getting your digital therapeutic on the market in the USA.
What is DTx?
Digital therapeutics (DTx) “are a health softwares intended to treat or alleviate a disease, disorder, condition, or injury by generating and delivering a medical intervention that has a demonstrable positive therapeutic impact on a patient’s health”.
What’s more, DTx are clinical evidence based, meaning often there have been conducted clinical trials which were reviewed by the regulatory agencies.
DTx can either support or (in some cases) replace pharmacological therapy. If you need an example of DTx, think about mobile apps that help people after stroke rehabilitation.
If you want to ensure your software is a DTx, you can check out the flowchart prepared by the DTx Alliance. Moreover, you can read about DTx, digital health, and digital medicine in our article.
DiGA-like scheme in the USA
You have probably heard about the world's first DTx reimbursement system, Germany's DiGA. Also, you may have learned about similar European systems – such as the mHealth Pyramid in Belgium. We describe DTx reimbursement schemes in Europe in a separate article.
Nonetheless, what is the situation in the US? Is there any DiGA-like scheme? The US government is undertaking DTx certification and reimbursement as in many other places worldwide. Let’s start from the beginning.
Who and what regulates DTx in the USA?
The body responsible for DTx certification in the United States is the FDA (U.S. Food and Drug Agency). They are the ones who provide society with information on whether or not a particular product is safe to use. To be more precise, the specific organisation in the FDA responsible for medical devices is the Center for Devices and Radiological Health (CDRH).
One of the most crucial documents which regulates medical devices is The Medical Device Amendments of 1976 to the Federal Food, Drug, and Cosmetic Act, which created the three-class, risk-based classification system for all medical devices. This and other general and permanent rules published by the Executive departments and agencies of the Federal Government are all gathered creating The Code of Federal Regulations (CFR).
Title 21 of the CFR is reserved for rules of the Food and Drug Administration. We encourage you to read Title 21 CFR Paragraph 860.3 regarding medical device classification if you consider getting your DTx on the American market.
What is the Access to Prescription Digital Therapeutics Act?
When you read about DTx reimbursement in the US, you might come across the Access to Prescription Digital Therapeutics Act. What is that?
This proposal of regulation states how the federal insurance system (e.g. Medicare and Medicaid programs) should cover Prescription Digital Therapeutics. The Access to Prescription Digital Therapeutics Act aims to improve Americans' lives and allow them easy access to Prescription DTx.
However, you should know that this bill is not a law at the moment. The Access to Prescription Digital Therapeutics Act was introduced in the Senate in March of 2023. Thus, we must be patient while waiting for other information on this bill.
What are the requirements for digital therapeutics in the USA?
In the Access to Prescription Digital Therapeutics Act, we can read that Prescription Digital Therapeutics must fulfil some requirements. Although this bill is not in law, the following points are typical for common definitions of DTx. What are they?
It must be a product, device, internet application, or other technology.
It primarily uses software to achieve its intended result.
Serves to prevent, manage, or treat a medical disease, condition, or disorder.
It is cleared or approved by the FDA (we will discuss the difference later).
It’s safe to use without professional assistance.
Moreover, specific regulations must be met, depending on the class of the medical device.
DTx and data protection in the USA
One of the requirements your DTx must meet is national specific data protection requirements which, depending on the medical device and the methods of transmitting, receiving or recording health information, may mean compliance with HIPAA (Health Insurance Portability and Accountability Act).
HIPAA is a national standard for protecting patients’ information. Consider it the American equivalent of the GDPR (General Data Protection Regulation) functioning in the European Union. You can read more about HIPPA and GDPR in our article.
Why should your app comply with HIPAA? Because as patients use your app, they might share information on their health with you. Thus, you process protected health information (PHI) which fall under the HIPAA regulation.
Quality Systems in the DTx development
The other thing a DTx manufacturer must consider is the quality system of manufacturing their product, which is described in the Quality System Regulation (21 CFR Part 820).
Quality System Regulation Part 820 is not the same as ISO 13485; however, there are plans for harmonisation of the American Regulation with ISO standard. Nevertheless, it is worth noting that ISO 13485:2016 is not a law or regulation and is voluntary, while FDA 21 CFR Part 820 is mandatory for medical device distribution in the United States.
What are the quality system requirements of the FDA? They are divided into three main categories:
management responsibility – management responsibility should include actions about policies, resources, quality planning and establishment of quality system proceduresand instructions,
quality audit – “manufacturer shall establish procedures for quality audits and conduct such audits”,
personnel – “manufacturer shall have sufficient personnel with the necessary education” (source: eCFR).
You can read all about the quality requirements, controls, and more in the Quality System Regulation.
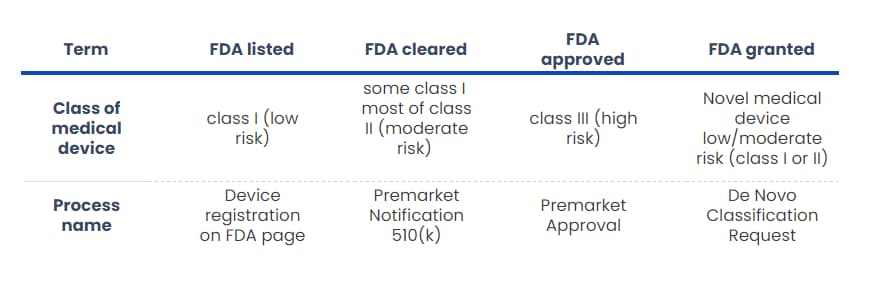
FDA listed, FDA cleared, FDA approved, and FDA granted – what’s the difference?
Before discussing getting your DTx reimbursed in the USA, we must describe four terms you might come across when reading about medical devices. There is a distinction between FDA listed, FDA cleared, FDA approved, and FDA granted, which you should know.
First, FDA listed (or registered) means that the medical device has been registered in the specified FDA’s database. It’s something all the manufacturers of medical devices must do; however, you will mostly hear about it in terms of medical devices of class I.
Those DTx are of low risk. Thus, there is no need to go through a complicated submission. A manufacturer simply registers their product once it meets the specified conditions (i.e. Quality Management System).
FDA clearance is intended for class II (and some class I) medical devices, which don’t have high usage risks. Usually, our device is substantially equivalent in terms of safety and effectiveness to a predicate medical device that has already been placed on the market. Thus, there is a unique way of getting FDA clearance, named 510(k), which we will describe later in this article.
On the other hand, FDA approval means that “the agency has determined that the benefits of the product outweigh the risks for the intended use”. FDA approval is used with medical devices of class III, and it is the most restrictive form of introducing a medical device to the US market. Before approval, the FDA evaluates scientifically the results of tests and clinical trials done by the manufacturer to ensure the safety and effectiveness of medical device. Bear in mind that the FDA only evaluates, not conducts any research!
Last but not least, the FDA granted is a relatively new term. Let’s say you have a medical device that is so novel that it was not previously classified. In other words, no predicate exists on the market. Normally, in such a case, your novel medical device would be classified as a high-risk class III. However, the risks of using your device are not that high compared to other devices.
This gives the opportunity to request FDA to review all safety information of your product through the “De Novo” process to be the device granted for marketing approval. Finally, your medical device might be classified as a Class I or II.
As you can see, the most important thing to do is establish the type of DTx you create. It would help if you answered the question, “What’s the level of its risk?”. You can read a little more about this on the FDA website.
Four ways of getting your DTx on the US market
Once you classify your DTx, you should establish the most suitable way of getting your DTx on the American market. There are four ways:
Register and List
Premarket Notification 510(k)
De Novo Classification Request
Premarket Approval
Which one is proper for your product? Let’s get into it.
How to register and list a medical device?
Most medical devices of Class I and some of Class II don’t have to go through the rigorous, complex submission process. The list of medical devices that are “510(k) exempt” is quite long.
If you manufacture any of these products, you will only be obligated to register your establishment and list manufactured products in the FDA database. You can find a step-by-step tutorial on registering and listing on the FDA website.
Take notice
A manufacturer must undertake this action even if they go through 510(k) or PMA. They will have to register their product after getting FDA clearance or approval.
What is Premarket Notification 510(k)?
510(k) is dedicated to some class I and most class II medical devices. 510(k) means a manufacturer must prove that there is a predicate device on the market that has already been stated as safe and effective.
Although it is the most common review pathway, bear in mind that the completion of the 501(k) process usually takes about 177 days.
What is a predicate device?
A predicate device is equivalent to your DTx regarding intended use, technological characteristics, and performance testing. A predicate device has already been checked regarding its safety and effectiveness.
As we read on the FDA website, you should prove that your DTx and the predicate device either:
“Has the same intended use and the same technological characteristics”.
or“Has the same intended use, different technological characteristics and does not raise different questions of safety and effectiveness and the information submitted to FDA demonstrates that the device is as safe and effective as the legally marketed device” (source: FDA).
You can read more about the review process of the 510(k) submission in the following document.
What’s a Premarket Approval?
Premarket Approval (PMA) is dedicated to Class III devices, which are of high risk. If you want your DTx approved by the FDA, you must present “scientific evidence demonstrating reasonable assurance of safety and effectiveness for the device’s intended use”.
PMA is highly rigorous as the FDA wants to ensure the device is safe for its users. Thus, there is a need for an “in-depth review of scientific and clinical data”, which can be either nonclinical laboratory studies or clinical investigation.
The list of documents which should be included when going through the PMA process is quite long (e.g. device description, foreign and US marketing history, summary of clinical and nonclinical studies). If you want to learn more about it, we recommend reviewing a document that states all the necessary information.
The PMA review process consists of four steps:
An acceptance and filling review (FDA checks if the application is complete and contains true statements).
A substantive review (in-depth review of the application which might result in the manufacturer updating their application).
A panel review (review by an outside panel of experts).
Notification of FDA’s final decision.
You can find all the medical devices which went through PMA in the database on the FDA website.
What is De Novo Classification Request?
We have already mentioned the De Novo Classification Request (or De Novo Process), but let’s break it into pieces, shall we?
De Novo Process is suitable for novel devices with low to moderate risk which don’t have a “predicate device”. Because of this, your DTx would be considered a Class III despite being low risk.
However, if you go through the De Novo Process, the FDA can provide you with marketing authorisation as a class I or class II. What’s more, your DTx can then serve as a predicate device for other 510(k) submissions.
When to apply for De Novo Classification Request?
On the FDA website, we read that you can do it in two situations:
If you have applied for 510(k) and the FDA responds that there is no “predicate device”.
If you establish that there is no predicate device and 510(k) isn’t a suitable way for you.
What are the requirements for the De Novo Process?
There is much information you are supposed to include in the De Novo Classification Request, such as:
Administrative Information (device’s intended use),
Device Description,
Classification Information and Supporting Data (why DTx should be a Class I or Class II device, clinical data, and more) (source: FDA).
You can find an Acceptance Checklist for De Novo Classification Request here. In this document, there is a list of questions you have to answer if you want to go through the De Novo Process.
Where to find DTx cleared and approved by the FDA?
To our knowledge, no database is specific to DTx cleared and approved by the FDA. However, there are databases for both:
510(k) Premarket Notification medical device,
medical devices under the De Novo process.
Those are lengthy lists of all the medical devices cleared or approved by the FDA. Thus, you will find there are many devices, not necessarily DTx.
However, thanks to the research conducted by Phan, Mitragotri, and Zhao, we can assume that until December 2022, at least 23 DTx were cleared or approved by the FDA. To name some of them:
Nighware – an app which uses Apple products helps with insomnia caused by PTSD (post-traumatic stress disorder),
EndeavorRx – a game assisting children with ADHD,
PropellerHealth – an app for people with asthma that helps track medication and educate a patient.
Is it possible to get your DTx reimbursed in the USA?
Let’s assume that you went through the process of having your DTx cleared or approved by the FDA. Is it now possible to get it reimbursed?
We have already mentioned the Access to Prescription Digital Therapeutics Act of 2023. If the bill passes, Medicare and Medicaid would have to include Prescription Digital Therapeutics in their programs.
Remember
Medicare is a “federal health insurance program for people who are 65 or older, certain younger people with disabilities, people with End-Stage Renal Disease”, and Medicaid is a health insurance program for low-income people.
However, for now, Medicare’s inclusion of DTx is “tied to specific conditions and requires evidence of clinical efficacy” and Medicaid “can consider fee-for-service product coverageor coverage via benefit programs”. It’s also worth noting that the Department of Veterans Affairs is trying to partner with healthcare facilities in order to prescribe DTx to veterans, e.g. to relieve their chronic pain.
And what about private insurance coverage? Well, it depends on the company. Some of them might include reimbursement for DTx; others won’t. For example, Highmark covers digital therapeutics for substance use disorder, sleep disorder, PTSD and chronic back pain. Thus, getting your DTx into private insurance coverage might require some negotiations.
What is CPT code for DTx?
When seeking information on DTx coverage by insurance companies, you might come across the term CPT code.
CPT (Current Procedural Terminology) codes allows standardisation in naming medical procedures and services. Those codes are assigned to services, procedures, etc. and enable insurers to figure out what amount to pay providers.
The CPT code for Prescription Digital Therapeutics is A9291.

What are the limitations of marketing DTx in the USA?
Of course, marketing DTx in the US is a complex task, and you may face some barriers. Some of these are representative of any country where you will attempt to reimburse your DTx; however, some are typical of the US.
Getting an FDA clearance/approval/grant
As you have already noticed, getting FDA clearance or approval takes some work. You have to take your time and gather the necessary documentation. Despite it being challenging, you can achieve this with the right team of specialists and a complex plan.Aversion to the risk of physicians
Unfortunately, not all healthcare professionals are knowledgeable about DTx. Although more and more people are learning about these solutions, there is a risk that some doctors will be sceptical about prescribing this type of medication to their patients.
As a result, you may need some support from healthcare professionals. If this is the case, it is worth looking into ways of educating and reaching out to doctors.Lack of accessibility
Although more and more DTx approved or cleared by the FDA are being developed, the reimbursement problem for these devices remains significant as access to DTx in the US is quite limited. However, it is worth remembering that the US government is taking steps to make DTx more accessible.
How to get through the process of marketing DTx in the USA?
As you read this article, getting your DTx on the American market is complex and demanding. If you decide to undergo this process, you will have to prepare all the necessary documents and research (if required).
However, even during preparations for submission, the FDA is helpful. You can schedule a meeting with them and request feedback on your submission. Thus, they will pinpoint everything you want to work on.
We’d also be happy to get in touch with you and talk about the reimbursement process in Europe and the United States and your plans for marketing a DTx you manufactured.
Find out how DTx works in Europe



