
What is DTx - from idea to certification
11 min read
Contents
Since DTx is subject to MDR, you need to plan its development carefully. Building such an app has benefits, such as providing the patients with a meaningful healthcare innovation and challenges, like a time-consuming certification process. In this article, we’ll tell you all about it and give you tips on preparing to create a DTx app.
TL;DR
Digital therapeutics (DTx) are health applications that can be an addition to traditional treatments but can also be used independently, replacing conventional medicine. They undergo rigorous clinical and regulatory reviews for safety and efficacy.
DTx development involves planning for intended use, collaboration with medical professionals, user testing, and building an MVP. To launch DTx apps in the European market, you need to remember about compliance with regulatory standards, clinical evidence, and post-market surveillance.
In the future, DTx has the potential to transform healthcare by offering secure, affordable, and personalised digital health solutions, ultimately improving accessibility and changing disease management practices
What is digital therapeutics (DTx)
Digital therapeutics (DTx) is evidence-based health software intended to treat, prevent or manage a disease, disorder, condition, or injury by generating and delivering a medical intervention with a proven clinical benefit.
Medical specialists may prescribe DTx independently or alongside existing drugs, devices, or therapies to optimise patient care and health outcomes.
DTx products undergo rigorous clinical and regulatory reviews, ensuring the highest efficacy and safety standards.
Good to know
Digital therapeutics may seem similar to health & wellness applications, but it’s validated as a ”medical-grade” solution with more specific and rigorous requirements to launch. Digital therapeutics is a digital medicine field, a subset of the digital health area.
You can read more about the differences between digital health, digital medicine and DTx in our article.
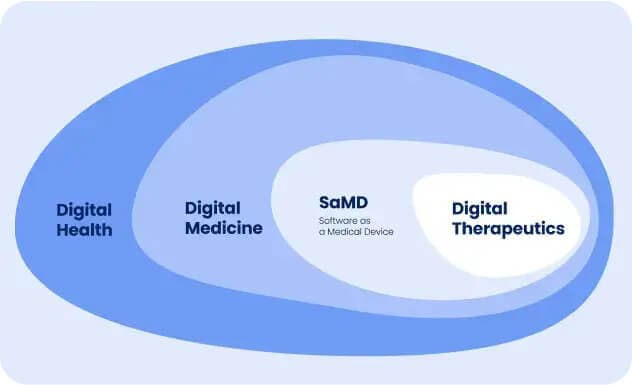
Digital medicine uses technology for medical treatments, while digital health involves a broader use of digital tools for healthcare and well-being.
DTx and SaMD
Digital Therapeutics (DTx) can also be described as Software as a Medical Device (SaMD).
SaMD refers to software intended for medical purposes, while DTx is evidence-based software for treating, managing, or preventing diseases.
Therefore, most regulatory authorities treat digital therapeutics as a sub-concept of SaMD.
DTx and MDR
As previously mentioned, we can recognise the DTx as SaMD. This means that if you want to distribute the app on the European market, it must meet the requirements set by MDR (Medical Device Regulation).
DTx is obliged to comply with MDR requirements, including safety, performance, clinical evaluation and post-market surveillance. Once a DTx has successfully undergone the conformity assessment, it can be labelled with the CE marking. We’ll discuss the process of certification in further paragraphs.
DTx and traditional medicines: the differences
Digital Therapeutics aims to cure illnesses or/and support therapy based on medicines.
It’s possible to use DTx as a sole form of therapy.
The “competition” between drugs and DTx is caused by their different functions in treatment. Let’s take a look at some of them.
Pharmaceutical drugs have potential side effects and clinical challenges, including toxicity, allergies, and blood barriers. On the other hand, medical devices like DTx have risks related to patient engagement in the therapy process rather than physical concerns.
Medicine use requires physical interaction with them, like swallowing, while using DTx comes down to using the app’s interface. Therefore, medicines can cause side effects and clinical complications, while DTx concerns focus more on risks related to intended use.
Drugs are available for patients in the pharmacies but DTx can be found in the app stores, and their costs can be reimbursed by public and private health coverage plans. Remember that not every DTx requires a prescription.
Regarding development, DTx requires a shorter implementation time because they have a less engaging clinical trial phase. However, DTx and medicines require clinical evaluation and approval from regulatory bodies to ensure their safety and prove that they are effective.
Because DTx is a digital, not a physical product, it has lower development costs than pharmaceuticals since manufacturing facilities and material costs aren’t necessary.
In the case of DTx, the potential updates are available through easily applicable changes in software, while changing the chemical composition of drugs is more complicated and definitely requires more time and higher costs.
Considering the differences and similarities presented above, it’s important to notice that digital therapeutics may encounter an obstacle, such as a digital barrier. The patient’s age, level of digital skills and medical care habits can result in a lack of trust in DTx.

Characteristics of DTx
Since digital therapeutics are mostly software-based products, they’re subject to numerous globally accepted standards and local regulations. DTx products should comply with principles, including product safety, efficacy, quality, patient centricity, privacy, and ongoing clinical impact.
Take a look at other DTx facts:
Not every evidence-based digital product used in the healthcare industry is a DTx. To be called such, the product needs to treat, prevent or manage disorders or diseases.
DTx apps in general are available in the app stores, yet you will be required to enter an authorisation code from the medical specialist.
All DTx products protect patient data security or privacy – to launch a DTx on the market, it must comply with the security standards: GDPR in Europe and HIPAA in the US.
DTx apps are not social media, so we can’t expect entertainment. Despite user- friendliness, they’re designed to support therapy and should be “dosed” during the right time durations.
Take notice
What’s also important about DTx features is their personalisation. While prescribing specific applications, the medical specialist considers the patient’s condition, course of therapy, preferences, and engagement. As a result, the patient receives a tool that enhances the therapy results and can be used in their own comfortable environment.
Categories and examples of existing DTx apps
Digital therapeutics is generally described as software-based but not software as such. It can fall into technological categories based on its intended use. The classification includes treating health disorders, controlling or preventing medical conditions, streamlining medications or treating medical ailments or conditions.
Here are examples of technologies in which DTx can be used:
Standalone apps: mobile apps that patients can download to their smartphones or tablets that often provide interactive and personalised interventions.
Example: ABAStroke, a digital health app for at-home neurological treatment of cognitive deficits for stroke survivors with minor motor deficits who require cognitive rehabilitation.Wearable devices and sensor-based systems: software integrated into wearable technology (smartwatches, fitness trackers) or using various sensors (accelerometers, gyroscopes) to monitor movements and collect data for therapy support.
Example: IEVA, a smartwatch that detects heart rate as well as environmental changes such as pollution, sun exposure, and noise levels.Online platforms: web-based DTx accessible through internet browsers, including knowledge repositories and therapeutical exercises.
Example: Selfapy, an application offering online personalised therapy programs on prescription.Connected Health: platforms to connect patients with healthcare providers remotely, often including remote monitoring.
Example: MS Sherpa is an app that supports patients with multiple sclerosis in monitoring the course of the disease and its symptoms and provides personalised neurological treatment.Virtual Assistants and AI: DTx can incorporate chatbots or virtual assistants to deliver AI- based therapeutic content, answer questions, and support users.
Example: Zanadio, an app for the treatment of obesity, offering chats with the trusted support team.Biofeedback Devices: DTx uses sensors to measure physiological parameters (e.g., heart rate, skin conductance) and provide feedback to patients to help them self-regulate their physiological responses.
Example: Mindfield eSense Skin Response, a small sensor to measure skin conductance using the microphone input of a smartphone or tablet.
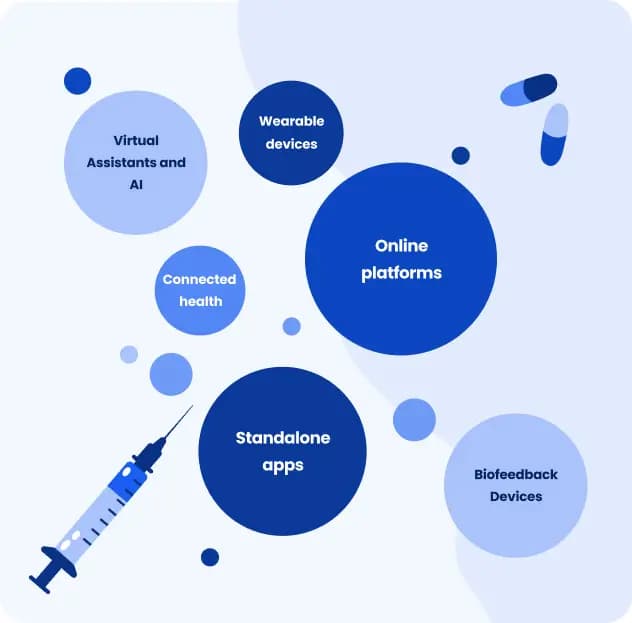
DTx may use numerous techniques to support therapy, from simple reminders and calculations (such as steps or calories) to results in interpretation, cognitive behavioural therapy or real-time monitoring.
Good to know
In many cases, different types of DTx can be used in one treatment to provide satisfying effects.
What are the benefits and challenges of DTx apps?
As we discussed previously, digital health applications and medicines seem competitive to traditional treatment, considering production costs and shorter clinical trial periods and implementation.
However, these factors are not the only benefits that DTx brings to modern healthcare. Then, why is it worth investing in building DTx? And what challenges may you encounter?
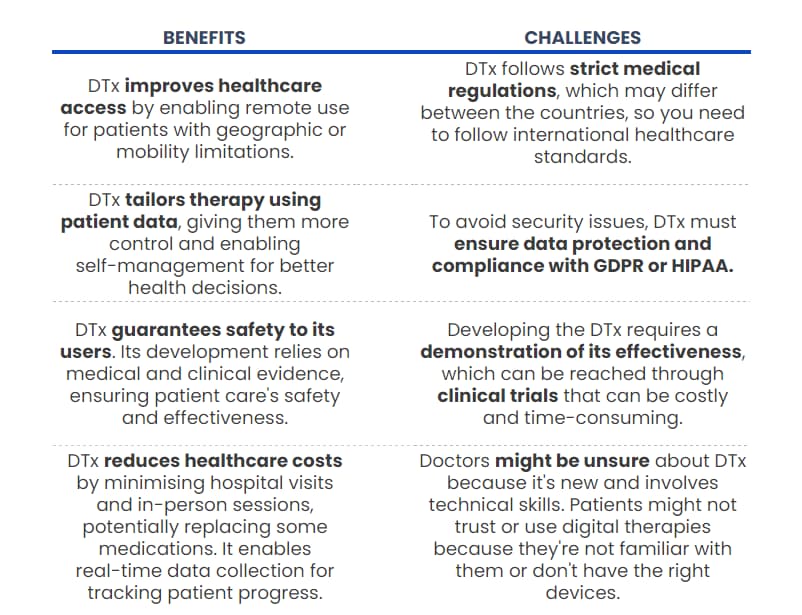
Despite these challenges, digital therapeutics have the potential to transform healthcare by offering innovative, patient-centred solutions that complement traditional medical treatments. And, as you can see, by deciding to build a DTx, you can provide healthcare with a life-changing product.

Risk classification of DTx apps
DTx on the European market is subject to the same MDR classification rules as SaMD. Its classification is determined by medical device risk classes, defined in Rule 11 MDR.
Let’s make a quick reminder of MDR risk classes:
CLASS I includes software considered to have the lowest level of risk that doesn’t require Notified Body certification.
CLASS IIa covers software having a moderate level of risk. It’s generally intended to provide information to make “low-risk” decisions with diagnosis or therapeutic purposes or monitor physiological processes.
CLASS IIb applies to software with a higher risk level than Class IIa. It’s intended to provide information for diagnostic or therapeutic decision-making that can have a significant impact, potentially leading to serious deterioration, surgical intervention, or monitoring vital physiological parameters with immediate patient danger potential.
CLASS III includes software considered to have the highest level of risk. It’s intended to provide information that is used to make decisions with diagnosis or therapeutic purposes that have an impact that may cause death or an irreversible deterioration of the user.
You can find the examples of Class I, IIa, IIb and III applications here.
Since, from the definition, DTx is software intended to treat, prevent or manage a disease, it will hardly ever fall into Class I, which doesn’t require the participation of the Notified Body in the certification process. Assessing the risk connected with the intended use of DTx, it seems rational that the minimum class for this type of product will be IIa in most cases.

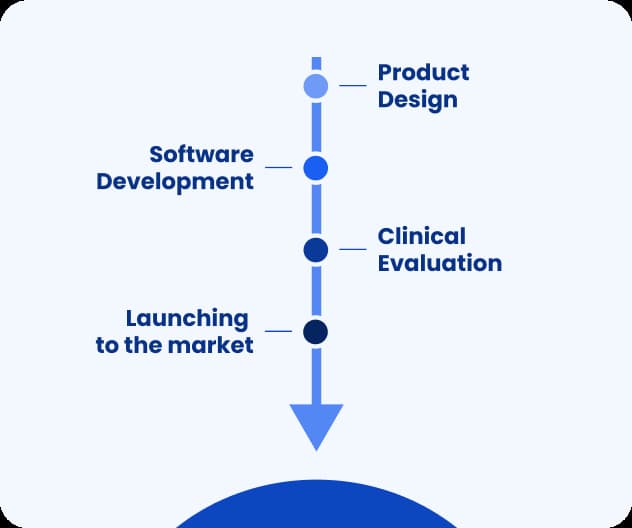
Launching DTx to the market: the process of product design, development, and certification
Let’s imagine the situation: you’re a medical software manufacturer, have an idea for the application, and know how it should help the patients. But what’s next?
Think about the features
First, you may want to consider your app's intended use, business plan, technologies, and certification needs. You can do it with your in-house team or find a contractor. If you decide on the second option, we suggest looking for a company with ISO 13485 certification, as it will likely be a critical supplier.
DTx plays a double role in the healthcare industry: it has to be safe for the patient and present high efficacy in therapy, and, at the same time, it has to appeal to consumers with ease of use and intuitiveness. That’s why the digital health software development process begins with the Product Design phase.
Product Design
The Product Design phase is necessary to begin software development and provide the development team with clues on how the software should work. It’s a great time to conduct user research and user testing.
DTx application’s interface has to consider the physical condition of the patients and any possible disabilities related to the treated dysfunction. Some apps link to consumer devices like wearables, which creates a need for inclusive interface development for various devices.
Good to know
When you decide to work on Product Design with an outsourced team, the process usually consists of a workshop, during which you’ll discuss the details of your app with the UX and development team, the necessary medical regulations and estimate the time and budget for the development.
Software development
Software development begins with building MVP (Minimal Viable Product) – it’s the first version of a healthcare app with only the most important features.
Once the MVP is accepted, the developers work on the product to achieve full functionality and eliminate bugs. The team analyses software requirements, works on the architectural design, unit implementation and verification, and carries out integration and system testing. The final step is software release – now the software is ready to use.
Developing regulated software must comply with various ISO and IEC standards (especially ISO 13485, IEC 62304, and IEC 62366), including requirements for risk analysis, extensive documentation and a strong emphasis on quality assurance processes.
Tip
You also need to know that digital health software developers don't create DTx applications alone but collaborate with doctors and medical units to ensure usability. Patients also participate in the validation process. The input from the intended user of the application is the crucial one.
Read more about the process of software development in our article.
Certification
When the product is ready, it goes through a rigorous clinical evaluation to demonstrate its safety and efficacy. It should generate clinical evidence that aligns with the regulatory requirements in Europe.
Take notice
If your product is innovative and has no equivalent in the market, you’ll need to conduct clinical trials to get clinical data confirming that the product is safe and effective
After clinical investigations, the app undergoes regulatory approval. The Notified Body (you need them only when your app is classified as IIa or higher) reviews the project documentation, proper classification of DTx, intended use and clinical evaluation regarding MDR regulations.
The positive result of the conformity assessment allows you to register your medical device and place the CE mark on it, which confirms that it meets regulatory and safety requirements.
Once you have obtained the necessary approvals and certifications, you can launch your DTx product in the European market. Be ready for post-market surveillance: establish procedures including monitoring and reporting adverse events and implement security measures compliant with GDPR to protect user data.
Read more about launching your DTx in our articles in the European and US markets.
If you need an experienced company to help with product design, development, and certification, here we are!
Why is clinical evidence so important in building DTx apps?
The primary reason for carrying out a clinical investigation is the lack of clinical evidence for the product's clinical effectiveness and safety. The software should aim to “prevent and reduce risks, errors and harm that occur to patients during provision of health care” as defined by the WHO. Results of clinical investigations help identify and mitigate potential risks, adverse events, or unintended consequences of using the app.
It also demonstrates that the DTx app effectively achieves its intended therapeutic goals, proving it can produce meaningful health outcomes.
Healthcare providers and patients need assurance that the DTx app they are using is backed by clinical evidence. Clinical data builds trust and confidence in the effectiveness and reliability of the app. Making solid clinical evidence for DTx apps can improve public health. Effective DTx interventions can address chronic diseases and public health challenges on a broader scale.

What’s the future of DTx?
The future of DTx and digital health direction in Europe and the US depends on developing patients’ and medical professionals’ awareness and improving technologies. So, does it have a chance to become one of the digital health trends?
Increasing awareness
Currently, we observe that the issues of digital therapeutics are noticed and are definitely going to develop. The institutions advancing DTx worldwide are Digital Therapeutics Alliance (DTA) and DTx Estonia. They aim to increase awareness and use of clinically tested DTx through education and collaboration, enhancing health outcomes for patients, clinicians, and policymakers with effective tools.
A phygital patient
Solutions in digital health applications can lead to a new type of patient – a phygital patient (physical-digital) who wants to use digital medical applications to improve their health. For patients who suffer from the side effects of drug therapies or for whom the existing treatment doesn’t bring satisfactory results, digital therapeutics apps can be an effective alternative.
Personalisation
In the future, digital solutions will be a vital element of a health ecosystem that meets the needs of the patient, who expects secure digital health and well-being solutions that are affordable and reimbursed by the healthcare system. With the development of DTx covering more and more diseases and disorders, with modern wearables, sensors and AI assistants, the apps will become natural choices while personalising the therapy.
Availability
DTx, introduced on a broad scale, can also change the availability of medical services because it’ll reduce the need for regular on-site treatment and help minimise the expenses and waiting time for an appointment. Moreover, digital therapeutics have a real chance to change disease prevalence in the future and become a tool not only for adult patients but also for children and their parents – convenient digital therapy will be able to minimise innate disorders and prevent future ones.
Have you thought about building your own DTx? Or maybe you’ve got an idea and don’t know how to start the development? Let’s talk about it!
Learn more about DTx reqirements in Europe



