
What is the intended use of the medical device?
4 min read
Contents
Writing down your medical device's “intended use” is one of its most crucial certification steps. Today, we will explain what it is and how to differentiate it from similar phrases.
What does intended use mean?
Medical Device Regulation is a document which states the rules for certifying medical devices (including medical device software) in the European Union. One of the pieces of information you will find there is a definition of the intended purpose.
The intended purpose is “the use for which a device is intended according to the data supplied by the manufacturer on the label, in the instructions for use or in promotional or sale materials or statements and as specified by the manufacturer in the clinical evaluation” (MDR Article 2, paragraph 12).
However, if you read carefully into Article 2 of the MDR, which contains all the key definitions, you will not find the term “intended use”.
Can we, therefore, conclude that “intended purpose” means the same as “intended use”?
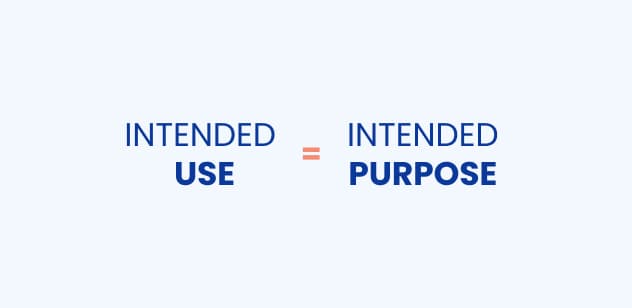
Is the intended use the same as the intended purpose?
Yes, the intended use and intended purpose relate to the same thing. Although, in the MDR, you will only find the term “intended purpose”, MDCG (Medical Device Coordination Group) states that the intended purpose and intended use are the very same.
However, some people state that there is a difference between those terms, which comes from the translation from English to the native languages of countries of the European Union.
Also, it would help if you kept in mind that the FDA (U.S. Food and Drug Administration) uses the term “intended use” when speaking about the aim of the medical device.
In a nutshell – intended use states what your medical device is supposed to do (including some extra information which we will describe next).
What are the components of intended use?
Although intended use is all about what the medical device is supposed to do, you can’t talk about this without including other aspects, such as:
medical conditions (e.g. illness),
intended patient population (e.g. adults/children),
intended user (e.g. a layperson or professional user with a medical background),
mode of action,
environment in which medical device should be used.
While writing down the intended use, remember to use clear, unambiguous language. You should explicitly state all the necessary details and ensure everybody understands them the same way.
Intended use – examples
We encourage you to check out how others have written intended use for their medical devices software. Where should you look for it? You will find it in the instructions for use of the medical device.
You can look for the medical devices software through the EUDAMED base (remember to choose MDR in the “applicable legislation” section and software in the “device type” section). Next, look for instructions for those medical devices on the internet.
We have chosen some instructions for CE-marked medical devices software to make it easier for you. You can find intended use examples below:
Intended use vs normal use – the difference
Intended use isn’t the only phrase you will come across when preparing the documentation of your medical device software. You will also read about the normal use. So, what’s the difference between intended use and normal use?
In the simplest terms, normal use is a broader concept than the intended use, including the medical aspect (treating, alleviating, etc.) and the medical device's maintenance, transportation, and storage.
You can find more information on normal use in the IEC 62366.
The difference between intended use and indications for use
The other thing you will have to think about when preparing documentation for CE marking is “indications for use”. Despite it sounding similar to intended use, those are two different things.
In a guide by MDCG we read that indications for use “refer to the clinical condition that is to be diagnosed, prevented, monitored, treated, alleviated (...) by the medical device”. Basically, indications for use are the reasons for using medical device software.
Remember
Not all medical devices will include the indication for use; for example, it’s not needed for medical devices with the intended purpose of sterilisation of other devices.

Writing down the intended use should be the first step of the medical device software development. Doing so can clarify what your product can do, but it also helps with the process of CE-marking.
First, by writing down the intended use, you can learn if your product meets the definition of a medical device (MDR, Article 2, paragraph 1). Second, intended use allows you to establish the risk class of your product (there are four classes – Class I, Class IIa, Class IIb, and Class III). Third, once you know the class, you can tell how your clinical evaluation will look, for example, if you need clinical investigation.
So why is it important to write down the intended purpose at the beginning of medical device software creation? You can roughly establish how long the certification process will take and how costly it will be.
At Revolve Healthcare we can help you with writing down your intended use according to MDR but also FDA standards. Our Regulatory Affairs Team will happily guide you through the process and answer any questions during the Compliance Audit. Feel free to contact us and schedule a free regulatory consultation to learn how we can help you.



